Memory Without Neurons: New Evidence of Learning in Single Cells
How Single-Celled Organisms Learn and Remember Without Brains—Rethinking the Quantum Biology of Memory
Traditional scientific models have long assumed that memory and learning require sophisticated neural structures. Complex brains with billions of interconnected neurons were thought to be the exclusive domain of information storage and adaptive behavior. Yet consider Stentor roeseli, a single-celled organism with no brain, no neurons, no synapses—that nonetheless demonstrates genuine learning by systematically changing its behavioral responses to repeated stimuli.
When researchers at Dartmouth exposed these trumpet-shaped protists to irritating stimuli, the organisms progressed through increasingly vigorous defensive behaviors, effectively “changing their minds” about how to respond. This remarkable finding exemplifies how recent interdisciplinary research is systematically dismantling the narrow view that cognition requires neural tissue, revealing that memory might be a far more fundamental and widespread phenomenon in living systems.
The Need for a Paradigm Shift in Understanding Biological Cognition
Conventional neuroscience has developed detailed mechanistic accounts of memory formation—from the Hebbian principle of “cells that fire together wire together” to the molecular processes of long-term potentiation (LTP) that strengthen synaptic connections. Yet these mechanisms describe how neural systems implement memory without addressing what memory fundamentally is or why it should be confined to neural tissue. If computational functionalism holds that cognitive capacities “emerge” once neuronal networks reach a threshold of computational complexity [1, 2], then accumulating evidence of memory-like behaviors in unicellular organisms—life forms with no neurons or synapses whatsoever—challenges a key assumption of this paradigm: that cognitive functions arise only at the level of complex cellular computational architectures, rather than, for example, within subcellular cytoarchitectonics.
This gap between mechanism and explanation, combined with the surprising discovery of learning in brainless organisms, suggests we may need to reconceptualize memory itself. Rather than viewing cognitive-like memory as an exclusive property of neural networks, we might consider it a more fundamental capacity of living matter—one that could be rooted in subcellular processes, including quantum-scale dynamics within molecular architectures. In this article, we explore evidence from single-celled organisms that points toward this radical possibility, and consider how frameworks like quantum spacetime memory networks might provide a deeper explanation for the widespread distribution of memory across scales of biological organization.
Spacememory: A Theoretical Foundation for Scale-Free Cognition
In a companion article, we explored the idea of quantum states of brain neurons leaving trace imprints in the fine-scale structure of spacetime and remaining entangled across their worldline such that information from any spacetime coordinate can be recalled. The idea of memory being stored in spacetime has seen recent empirical support via experiments with quantum computers, which we discussed in the article Space-Memory Experiments: Quantum Memory Matrix Results Explained. So, the idea of spacememory, first proposed by physicist Nassim Haramein, is beginning to be more fully understood as it moves from theoretical foundations in the holographic principle and gravitational wave memory effect to empirical support like that observed in the quantum memory matrix experiments and we find mounting evidence for the intriguing possibility that may be an integral aspect of the scale-free cognition that is observed in living organisms [3].
For example, in our work we described how a postulated memory effect in quantum states—those that occur in a highly orchestrated manner within molecular architectures of the neuron [4]—might be the basis of cognitive memory [5]. Since these dynamics are occurring at the subcellular level, cognitive memory may not be confined exclusively to large complex nervous systems but found distributed in many cellular systems just as hereditary memory and morphogenetic memory is carried by every cell. In fact, evidence of cognitive-type memory capabilities in unicellular organism—life forms with no neurons or synapses—would be observational evidence of this model.
It is salient then that studies are revealing how unicellular organisms can exhibit behavior suggestive of learning and memory. These surprising findings provide supporting context for the idea that memory could be a fundamental process of living systems, perhaps rooted in the very architecture of nature—imprinted into the quantum geometrodynamic circuits of spacetime—rather than an ability exclusive to complex brains.
A Brainless Organism That “Changes Its Mind”
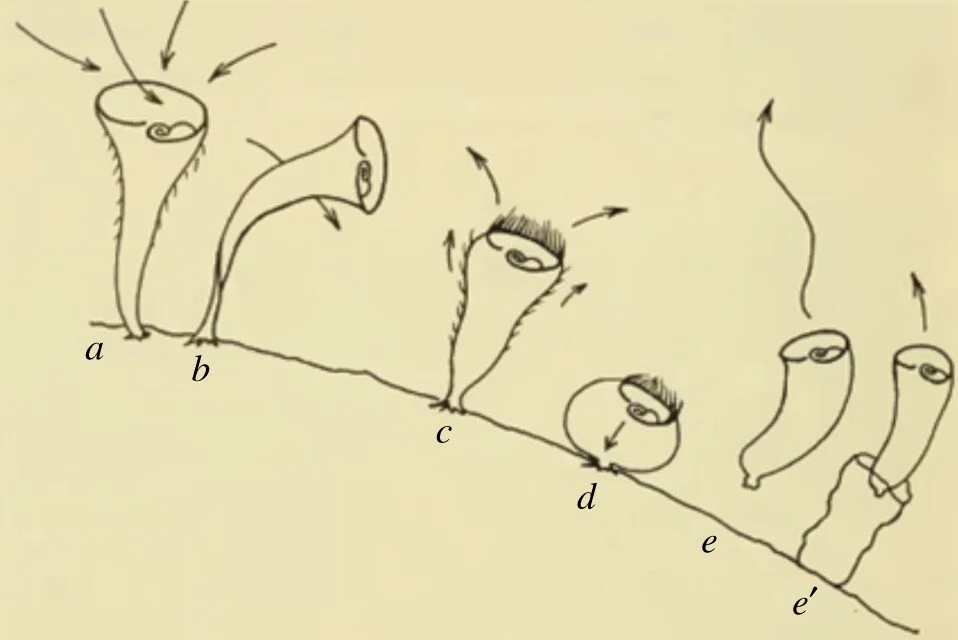
One of the most eye-opening cases of single-cell learning comes from Stentor roeseli, a trumpet-shaped freshwater ciliate. Over a century ago, zoologist Herbert S. Jennings reported that S. roeseli responds to irritation with a hierarchy of behaviors [6]: first gently bending away, then reversing its beating cilia to sweep the irritant aside, next contracting into a little ball, and finally detaching and swimming off if the annoyance continued (Figure 1).
Figure 1. Stentor roeseli in action: (a) at rest, (b) bending away from an irritant, (c) contracting into a ball, and (d) detaching from its anchor to (e) swim away. This single-celled protist demonstrates a sequence of avoidance behaviors escalating in intensity
In essence, this one-celled creature tries one tactic after another—almost like a tiny animal testing different escape strategies. Jennings’ 1906 observations were met with both fascination and skepticism, especially after a later attempt (in 1967) failed to replicate the results (possibly because it studied the wrong species). For decades, the idea that a single cell could show such complexity was largely written off as an anecdote of early science.
Then in 2019 a team from Dartmouth College, Cambridge, and Harvard Medical School decided to resurrect this old experiment with modern tools. They painstakingly obtained true Stentor roeseli specimens (even wading into ponds and golf course puddles to find them!) and designed a gentle micro-flow system to irritate the cells with tiny polystyrene beads [Modern Technology and Old-Fashioned Legwork Solve Science Mystery | Dartmouth].
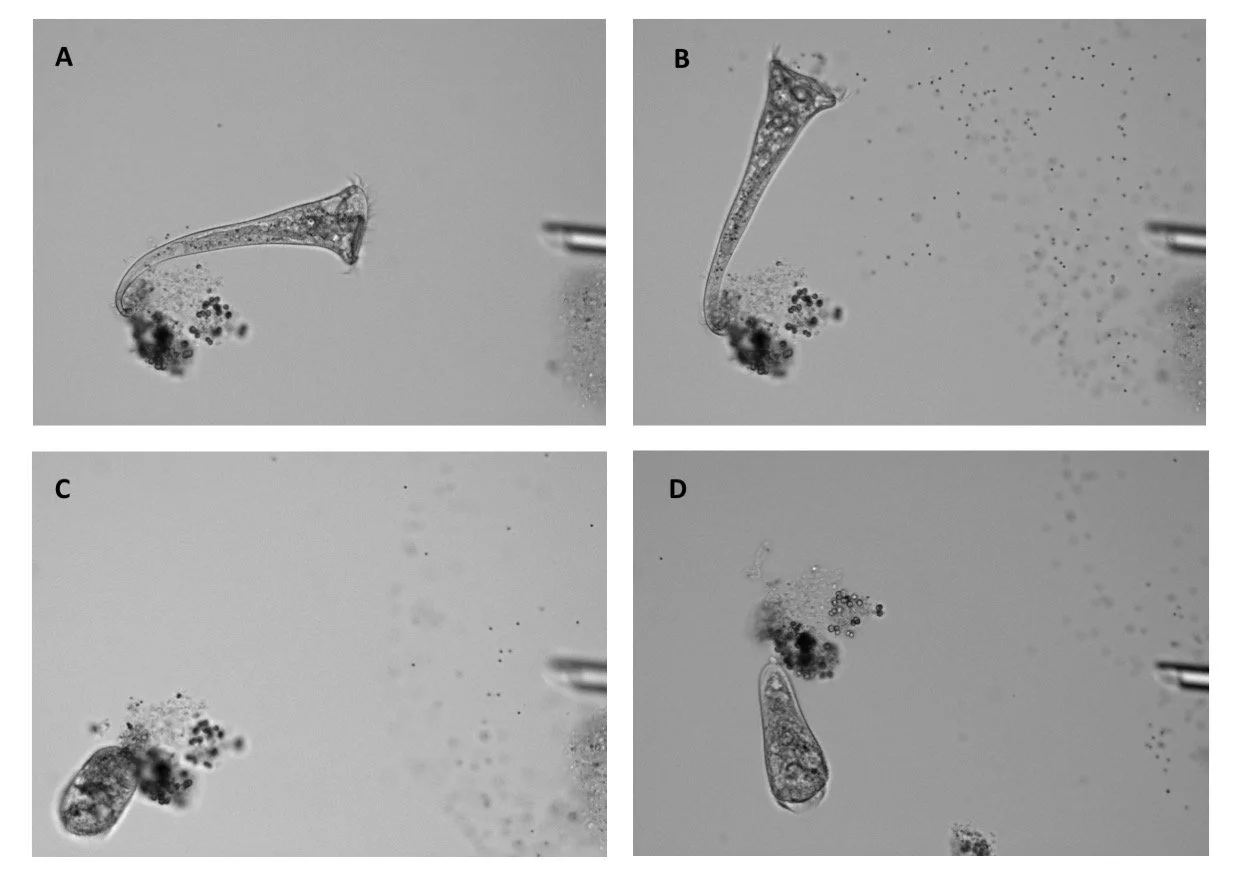
The results confirmed Jennings’ century-old claim. Under the microscope, Stentor typically reacted by bending away or altering its ciliary beating to avoid the beads, just as described long ago. If the irritating beads kept coming, the single cell would often contract its whole body into a protective ball, and sometimes it took the drastic step of detaching from its anchoring spot to float elsewhere (Figure 2). Not every individual cell followed the full four-step sequence each time, but statistically there was a clear pattern: simple avoidance maneuvers came first, and only if those failed did the cell “decide” to escalate to more extreme actions. Remarkably, when it came to those final two options (contract or detach), Stentor appeared to choose between them at random – almost like flipping a coin.
Figure 2. New research may have put to rest a century-old question on the behavior of the single-cell organism S. roeseli, shown here (a) resting, (b) bending, (c) contracting and (d) detaching in response to an irritant. Image credit: Joseph Dexter and Sudhakaran Prabakaran/Current Biology.
What astounded researchers is that S. roeseli has no nervous system or brain, yet it behaves as if it has a rudimentary decision-making process. “They do the simple things first, but if you keep stimulating, they ‘decide’ to try something else,” explained Dr. Jeremy Gunawardena, a systems biologist involved in the project [7]. In his words, Stentor roeseli can “change its mind” even without a mind: it acts flexibly based on past experience (continued irritation) rather than just a fixed reflex. This suggests some form of memory or internal state is at work. The cell seemingly “remembers” that the irritation has been going on and alters its behavior accordingly. The research team emphasized that single cells can be far more sophisticated than we usually imagine. If a brainless, one-celled blob in a pond can integrate information over time and modify its responses, then the seeds of learning and memory do not necessarily require neurons at all.
Other Unicellular Life with Memories
Stentor is not alone. In recent years, scientists have discovered that many organisms without brains show memory-like behaviors. From echinoderms (marine invertebrates comprising starfish, brittle stars, sea cucumbers, sea urchins, and sea lilies) [8] to plants [9].
A famous example is the slime mold Physarum polycephalum. Slime molds are bizarre single-celled amoeboids (often large and multi-nucleated) that can solve mazes, choose optimal nutrition, and even anticipate periodic events. In 2016, researchers demonstrated that Physarum can learn by habituation [10], the same simple form of learning psychologists study in animals. In their experiment, slime molds were presented with a bridge laced with a bitter or irritating substance (like quinine or caffeine) that the organism would normally avoid. At first, the slime mold hesitated, reacting strongly to the unpleasant stimulus. But after repeated trials, it learned that the irritant was harmless – it crossed the nasty bridge faster and with less hesitation each time. Once the offending chemical was removed, the slime mold returned to its original behavior, indicating it hadn’t just gotten used to moving faster in general, but had specifically adapted to that stimulus. This meets the key criteria for habituation: the creature adjusted its response based on experience, remembered that adjustment, and yet wasn’t permanently desensitized (it could recover its initial caution when conditions changed).
The researchers pointed out that basic learning requires at least three steps: a response to a stimulus, a memory of that experience, and a changed future behavior based on that memory. The humble slime mold accomplished all three steps without a single neuron in its amorphous-like body (to outside appearance, on the inside, at the molecular level, even a seeming amoeba has astonishing geometrically ordered organization).
Other single-celled protists, such as Paramecium and Tetrahymena, have long been suspected to exhibit learning-like behaviors as well. Early studies hinted that paramecia could be trained to respond to stimuli (for example, to navigate mazes or to expect food in certain locations), though these experiments remain contentious and difficult to reproduce. Nonetheless, a growing body of work is reconsidering these claims with modern methods and more rigorous analysis. There is even the highly revealing fact that unicellular organisms are affected by anesthetics [11]—compounds that disrupt whatever mechanisms are involved in memory formation in humans and result in a temporary loss of consciousness—demonstrating that the target of these compounds is not just the neuronal synapse and associated structures but something within like the microtubules of the cytoskeleton [12].
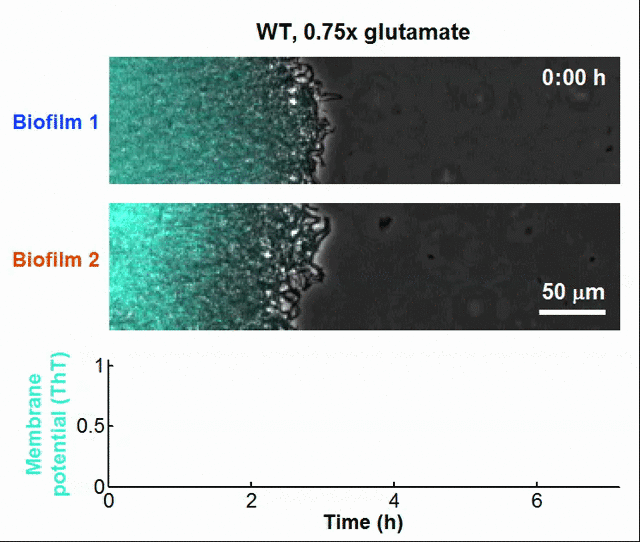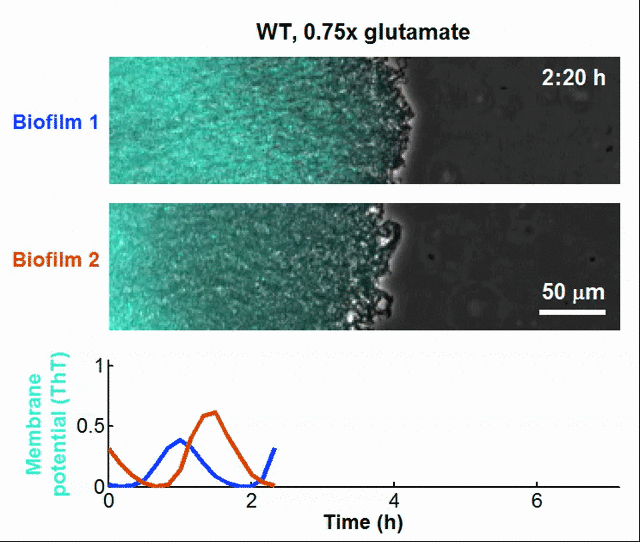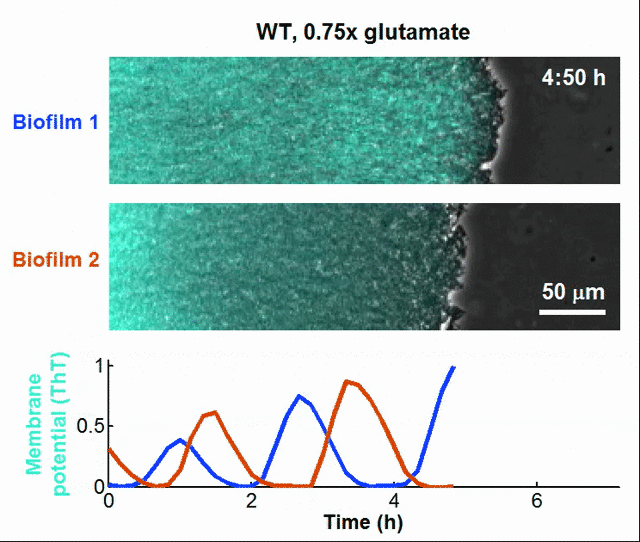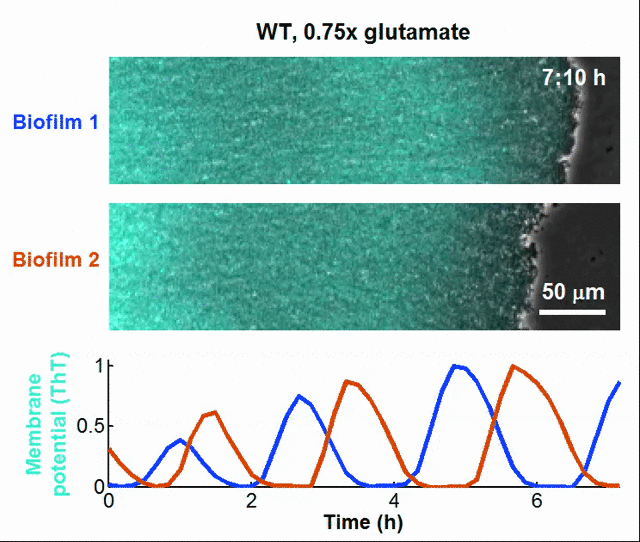
Even bacteria – often thought of as simple, hardwired organisms – might have rudimentary learning capabilities. For instance, bacteria can “anticipate” changes in their environment by tuning their gene expression based on past exposure to stressors, effectively showing a form of cellular memory. Biologists have even discovered that bacterial biofilms can communicate like neurons in the brain, such that bacteria achieves synchronization and coordinates collective actions at the population level through the utilization of electrochemical signals [13].
Global oscillations in membrane potential (ThT) in growing Bacillus subtilis biofilms.
Tikhonov, assistant professor of physics at Washington University said ““Evolutionary ‘learning’ is commonplace. For example, many organisms have evolved a circadian clock to follow the 24-hour day and night cycle. But evolution takes place over many generations. We show that bacteria could, in principle, do what we do: Learn correlations from recent experience and adapt their future behavior accordingly, even within their lifetime. Bacteria do not have brains, but we find that this kind of information processing can be achieved with a circuit that is not only simple, but similar to the circuits that bacteria are already known to have,” [14]. Tikhonov’s insight, following his research team’s empirical study, demonstrates that subcellular molecular mechanisms may be necessary and sufficient for whatever physics are involved in the formation of adaptive memory.
Building on this insight, biochemist Nick Lane has provocatively explored the foundations of awareness in unicellular organisms. In his groundbreaking work “Transformer: The Deep Chemistry of Life and Death” [15], Lane examines the complex electrical fields within bacterial membranes, posing a radical question: Could bacteria possess a rudimentary stream of consciousness?
Nick Lane at the Institute of Art and Ideas’ annual philosophy and music festival HowTheLightGetsIn exploring how “electricity creates consciousness” – https://iai.tv/video/electricity-creates-consciousness-nick-lane
While evidence like neuronal-like signalling and cognitive molecular circuits do not prove that single-cell organisms “think” like animals, it does suggest memory and learning have much deeper roots than we previously imagined. A range of brainless organisms seem capable of storing information about past experiences and using that information to guide their future behavior. In this light, memory may be less a function of complex neural networks, and more a basic strategy for navigating and adapting to one’s environment. In short, life at all scales seems to benefit from remembering the past. From one-celled amoebae to our own immune cells, the ability to adjust behavior based on prior experience confers a survival advantage, and evolution appears to have found solutions for this long before brains came onto the scene. Could it be that memory is far more intrinsic to nature than thought—extending beyond biology into something fundamental, like spacememory?
Memory Beyond Synapses: A Fundamental Process?
These discoveries fundamentally challenge our traditional notions of memory. We tend to equate “memory” with brains storing information in neural connections (such as strengthened synapses in a rat’s or human’s brain). But how do you explain a memory-like effect in a cell with no synapses and no nervous system at all? The answer may lie in subcellular mechanisms; mechanisms that are necessarily within the quantum domain, and as scientists Stuart Hameroff and Roger Penrose have expounded, involve quantum structure of spacetime itself [16].
Indeed, many researchers, including Hameroff, argue that single cells can store information through molecular changes—for example, by switching genes on or off, altering the concentrations of specific proteins, or even leaving physical marks on cytoskeletal elements such as microtubules [17]. In fact, some theorists suggest that biological memory might be encoded in molecules like RNA and/or in chemical modifications to DNA [18]. These changes could serve as a cellular analog of “writing something down” – a way for a cell to record that “I’ve seen this before” and thus respond differently in the future.
If unicellular organisms have found ways to recall past events, it hints that the roots of memory run deep. Evolutionarily, learning and memory likely predate the brain by hundreds of millions (if not billions) of years. The basic toolkit for forming memories may have existed in ancient single-celled ancestors as a solution to life’s challenges – a way to adapt to recurring stimuli in a changing environment. Complex brains and synapses may have later built on these primal memory mechanisms, rather than inventing memory from scratch. As one review noted, the mechanisms for memory storage in complex animals may have been inherited from much simpler organisms, given the efficiency and survival value of learning even at the single-cell level.
The capacity of unicellular organisms to recall past events confirms that memory and learning are foundational to life itself. These abilities are not late evolutionary innovations but intrinsic properties of biological organization—scale-free cognitive capacities that distinguish living from non-living matter. Memory, learning, and volitional behavior (adaptive, goal-directed responses to environmental challenges) represent the fundamental toolkit that enabled life to emerge and persist. These mechanisms existed in the earliest single-celled organisms as essential solutions to navigating changing environments. Complex brains and synapses built upon these pre-existing capacities rather than inventing memory de novo. The findings in unicellular organisms are not surprising anomalies but confirmations of a deeper principle: that agency, awareness, and adaptive memory operate across all scales of biological organization [19]. What we observe in Stentor and other microbes reflects the same fundamental processes that underlie cognition in complex nervous systems—evidence that memory is woven into the very fabric of what it means to be alive.
All of this lends intriguing support to the broader theory intimated earlier: that memory might not be solely a matter of neuronal circuits but could be something more fundamental – perhaps woven into the very fabric of biological organization, or even the fabric of spacetime itself. This is where ideas like quantum geometrodynamic memory networks come into play. Such pioneering theories propose that what we experience as memory could arise from deep physical processes—for example, quantum-entangled states within cell structures or electromagnetic vibrations that link molecules across time. In that view, the brain’s synapses might be more like the interface or receiver of memory, tapping into a deeper “storage” that exists at subcellular or quantum levels. The fact that single cells show a primitive kind of memory and decision-making makes this notion a bit less far-fetched. It suggests that memory is not an exclusive property of brains, but rather a general feature of life, potentially utilizing whatever mechanisms nature has on hand—from chemical networks in a one-celled organism up to quantum-entangled biomolecules in neurons.
Conclusion: A Universe of Memory
Watching a brainless microbe “change its mind” is a stark demonstration that intelligence and memory transcend brain matter. A one-celled Stentor dodging irritants or a slime mold learning to ignore salt challenges the assumption that memory requires billions of neurons. Instead, memory may be a universal strategy of life, present in different guises at all scales—an echo of past influence guiding future action. This line of evidence supports a provocative but profound idea: that memory might be a fundamental process woven into the architecture of nature itself, rather than a special circuit found only in brains. In other words, what we call “memory” could emerge from the deep interplay of physics and biology—from molecular marks to perhaps even spacetime quirks—not just from synapses and electrical pulses.
Such a perspective lights the way for fascinating interdisciplinary research. As scientists continue to uncover memory-like behavior in the simplest organisms, they inch closer to unraveling how information is stored and retrieved at the cellular level. Could the secret to memory lie in quantum vibrations of proteins, or in networks of molecules acting in unison, or in something even more exotic? We don’t yet know. But one thing is clear: memory is ancient, and it runs deeper than our neurons. From single cells defying our expectations to the frontiers of quantum biology, the quest to understand memory is expanding our understanding of life and even reality. And as we explore these depths, we inch toward a future where accessing a memory might one day be described not just in terms of firing synapses, but in terms of entangled spacetime – a fundamental connection across time and space that all living cells, in their own way, partake in.
In the end, the tiny Stentor in its pond and the memories in our human brains may share a common cosmic thread. Memory, it appears, is far more than a record in a neural network – it may be a basic property of living matter, a whisper of the universe’s own capacity to remember. And that is a thought as wondrous as it is exciting, inviting us to rethink what it truly means to remember.
References
1. G. Piccinini and S. Bahar, “Neural Computation and the Computational Theory of Cognition,” Cognitive Science, vol. 37, no. 3, pp. 453–488, 2013, doi: 10.1111/cogs.12012.
2. W.D. Brown, “The Consciousness Clash – The International Space Federation (ISF).” Accessed: Sep. 19, 2025. [Online]. Available: https://spacefed.com/biology/the-consciousness-clash/.
3. M. Levin, “The Computational Boundary of a ?Self?: Developmental Bioelectricity Drives Multicellularity and Scale-Free Cognition,” Frontiers in Psychology, vol. 10, 2019, doi: 10.3389/fpsyg.2019.02688.
4. S. Hameroff, “‘Orch OR’ is the most complete, and most easily falsifiable theory of consciousness,” Cognitive Neuroscience, vol. 12, no. 2, pp. 74–76, Apr. 2021, doi: 10.1080/17588928.2020.1839037.
5. W.D. Brown, The Memory Field: Could Quantum Biology Involve Accessing Information Stored in Space Itself? – The International Space Federation (ISF). https://spacefed.com/biology/the-memory-field-could-quantum-biology-involve-accessing-information-stored-in-space-itself/ (2025).
6. Jennings 1902 Am. J. Physiol. Legacy Content8, 23–60, doi:10.1152/ajplegacy.1902.8.1.23.
7. S. J. Gershman, P. E. Balbi, C. R. Gallistel, and J. Gunawardena, “Reconsidering the evidence for learning in single cells,” eLife, vol. 10, p. e61907, (2021), doi: 10.7554/eLife.61907.
8. C. A. Freas and K. Cheng, “Neuroecology beyond the brain: learning in Echinodermata,” Learn Behav, vol. 50, no. 1, pp. 20–36, Mar. 2022, doi: 10.3758/s13420-021-00492-3.
9. M. Gagliano, V. V. Vyazovskiy, A. A. Borbély, M. Grimonprez, and M. Depczynski, “Learning by Association in Plants,” Sci Rep, vol. 6, no. 1, p. 38427, Dec. 2016, doi: 10.1038/srep38427.
10. “Single-celled life can learn,” Nature, vol. 533, no. 7601, pp. 10–10, May 2016, doi: 10.1038/533010d.
11. M. B. Kelz and G. A. Mashour, “The Biology of General Anesthesia from Paramecium to Primate,” Current Biology, vol. 29, no. 22, pp. R1199–R1210, Nov. 2019, doi: 10.1016/j.cub.2019.09.071.
12. T. J. A. Craddock et al., “Computational Predictions of Volatile Anesthetic Interactions with the Microtubule Cytoskeleton: Implications for Side Effects of General Anesthesia,” PLoS One, vol. 7, no. 6, p. e37251, Jun. 2012, doi: 10.1371/journal.pone.0037251.
13. S. Manna, C. Ghanty, P. Baindara, T. K. Barik, and S. M. Mandal, “Electrochemical communication in biofilm of bacterial community,” J Basic Microbiol, vol. 60, no. 10, pp. 819–827, Oct. 2020, doi: 10.1002/jobm.202000340.
14. S. Landmann, C. M. Holmes, and M. Tikhonov, “A simple regulatory architecture allows learning the statistical structure of a changing environment,” eLife, vol. 10, p. e67455, Sep. 2021, doi: 10.7554/eLife.67455.
15. N. Lane, Transformer: The Deep Chemistry of Life and Death. W. W. Norton, 2022.
16. S. Hameroff and R. Penrose, “Orchestrated reduction of quantum coherence in brain microtubules: A model for consciousness,” Mathematics and Computers in Simulation, vol. 40, no. 3, pp. 453–480, Apr. 1996, doi: 10.1016/0378-4754(96)80476-9.
17. T. J. A. Craddock, J. A. Tuszynski, and S. Hameroff, “Cytoskeletal signaling: is memory encoded in microtubule lattices by CaMKII phosphorylation?,” PLoS Comput Biol, vol. 8, no. 3, p. e1002421, 2012, doi: 10.1371/journal.pcbi.1002421.
18. P. Marshall and T. W. Bredy, “Cognitive neuroepigenetics: the next evolution in our understanding of the molecular mechanisms underlying learning and memory?,” npj Science Learn, vol. 1, no. 1, p. 16014, Jul. 2016, doi: 10.1038/npjscilearn.2016.14.
19. W.D. Brown, The Volitional Agent Criterion: Understanding How Agency and Awareness Define Living Systems Across Biological and Artificial Domains. 2025. doi: 10.1234/ISF.VA_Criterion2025.